 Over 27,000 women have had problems with Essure, which is a coil that is placed in the fallopian tubes. The manufacturer, Bayer, announced it will stop selling Essure as of the end of 2018.
Over 27,000 women have had problems with Essure, which is a coil that is placed in the fallopian tubes. The manufacturer, Bayer, announced it will stop selling Essure as of the end of 2018.
In 2016, the Food and Drug Administration (FDA) required Bayer to place a “black box warning” and a Patient Decision Checklist on the Essure package.
A patient in the clinical trials for the device also alleged that she had pain from Essure. NBC News looked up the details of the clinical trials and found the report for that particular trial participant, had an “excellent,” response.
The FDA will continue to require Bayer to follow up to promote patient safety. Bayer has already stopped selling the device in Brazil, Canada, England, France, and several other countries.
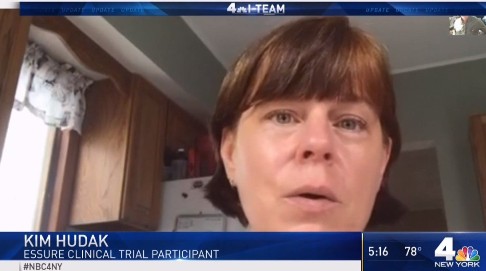
Also see New York Times article: Bayer Will Stop Selling the Troubled Essure Birth Control Implants.
